Uncontrollable bleeding is a major killer in battlefields. Almost 50% of the soldiers who have died, so far, died due to bleeding and about 30% of these were preventable deaths. The world over, militaries have made efforts to build products that ensure people don’t die due to bleeding. But India was lagging behind.
That was – until one man decided to change the statistic. What led to this was not his tryst with the army but with another rampant killer in India – road accidents, where again 40% of deaths are due to bleeding out.
It was in 2006, when Leo S. Mavely, a bio-engineering student commuting between South Delhi and Central Delhi witnessed a road accident that would dictate his fate like no other. A motorbike commuter had been hit by a bus close to Apollo hospital and lay profusely bleeding.
Leo reminisces, “Someone took him to the side and I helped him. But within few minutes, the place was empty. I was trying to get him to the Apollo Hospital which was hardly a kilometre away. He was bleeding profusely and I could do nothing. I literally felt this helplessness that I had nothing in my hand to control it – to stabilise him immediately.”
All Leo could do was wrap the wound with his jacket to stem blood flow but it was soon saturated with his blood. Luckily for the victim, Leo managed to get him to the hospital as the victim survived. But what also survived with Leo was that feeling of helplessness he had felt when the man’s life hung in the balance.
“That incident stayed with me. So when I started looking at projects at the NirmaLabs incubator, I wondered why we can’t develop a product that can immediately stop bleeding so that a patient can at least get few more hours of a chance to survive. The time, called the golden hour in road accidents, is very crucial because it still takes an ambulance 30-40 minutes in an urban setting and an hour or more in rural settings to reach the victim.”
And that’s how Leo’s fate as an entrepreneur was sealed. He was working on biopolymer-based devices during his college degree’s last year, he decided to take special permission to work at the NirmaLabs Incubator in Ahmedabad to pursue his search for the perfect product for a hemostat (blood flow stancher) in 2008.
Thus, Axio Biosolutions was born.

When Leo started it as a college project, he realised that the advanced wound care space had a lot of potential but very few products. “The idea was to build out a product platform based on a technology i.e. biomaterials technology.” For the first few years, Leo and his team built out the foundation, got the clinical studies going on. So it stayed in stealth mode till 2014.
The startup uses a natural biomaterial called Chitosan, extracted from shellfish, to make a sponge-like dressing that stops bleeding within minutes. After the technology got approval in India in 2011, it brought out the first line of products in India that can stop uncontrollable haemorrhage. Post that, in 2014, it received European clearances.
Axio decided to hit the market in a big way, thereon.
Hence, the company moved out of the incubator to set up its own manufacturing facility last year. It launched with the Indian Defence Forces and for last two years, through third-party manufacturing and has been supplying to all of the Indian armed forces – BSF, NSG, and CRPF. It has become more of a standard product in first aid kits when they go for any operation.
Thus, Axiostat has become the first product from India to address this need. And although the issue of death due to haemorrhage on the battlefield is potentially large, Leo and his team are slowly changing that.

Why Chitosan?
Leo attributes providence as to how he alighted upon the polymer Chitosan for making Axiostat. When the team was looking at different materials for manufacturing, he stumbled upon Chitosan and chose it. Interestingly, a lot of attention came to the material at the same time Leo was conducting his research, and now it has become a subject of interest in a lot of academic institutions.
“We were kind of fortunate to work on that material early on. It was a versatile material and it had a lot of properties which we could manoeuvre around,” says Leo.
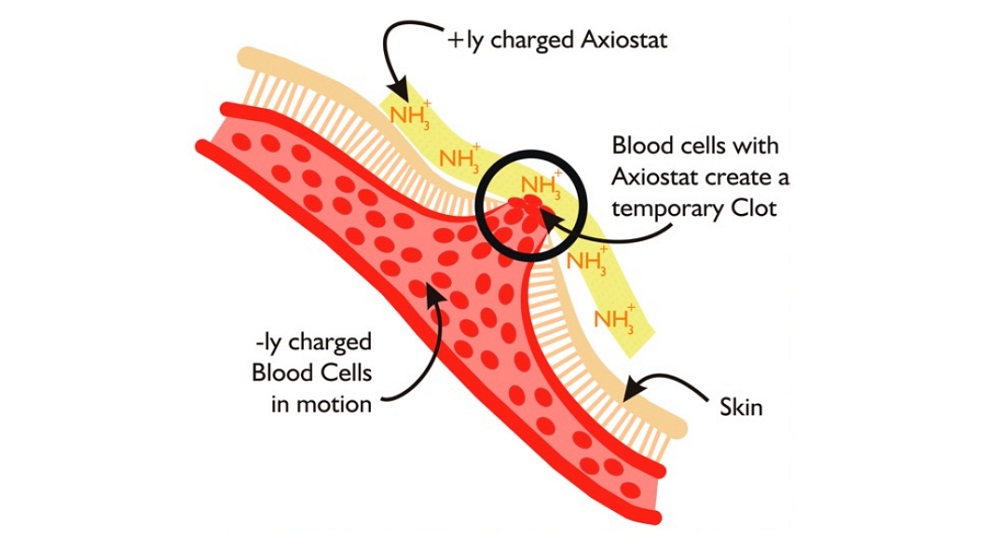
As a polymer, Chitosan is derived mainly from shellfish and is highly positively-charged. So, one can make it more positive or in other words increase its electrical density. It reacts with blood, which is primarily negatively charged platelets. Whereas RBCs carry a net negative charge. So the team utilised this property to develop a hemostat or the company’s first product Axiostat.
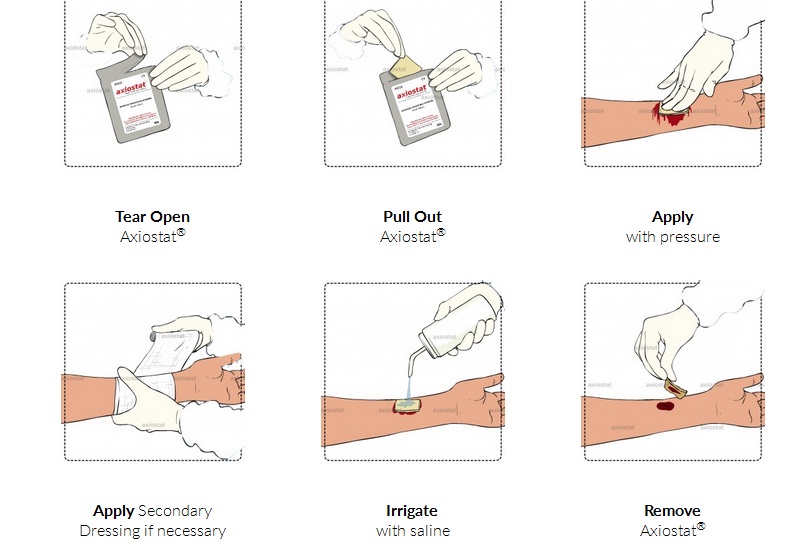
Axiostat is like a dry sponge but when applied with pressure on a bleeding wound, it immediately starts sticking to blood like fevicol due to charge-based adhesion. It clots the blood around itself. So if it is a profusely bleeding wound, it seals the vessel through an action of compression. It stays there for 48 hours if it is not removed.
To remove it, one can apply water or saline solution into it and the material turns itself into a gel which can be easily washed off. In addition, it also has antibacterial properties. The material is also used by the US army. It has developed a reputation over time as a biocompatible material right now.
70 Battalions On Board, 150K Units Shipped
Leo piloted the product for a while before swinging into business given it was a new product and his intention is to stay for the long term. So starting with the Indian armed forces, Axiostat is now used by almost 70 battalions. Additionally, it is supplied to major hospitals like Apollo, St John’s, Manipal, Breach Candy, Fortis and others. Besides, the team also works with certain public hospitals and government’s public health initiatives.
While Leo did not disclose revenues, the startup has an installed manufacturing capacity of 250K units in the Ahmedabad unit, with expandable capacity going up to 5 Mn units a year. So far, it claims to have shipped about 150K units and has a presence in around eight states in India and about 10 countries. Last year, it made inroads into the UK and this year, it has launched in the Middle East in a major way.
The range of products varies – from those for emergency use to those specifically designed for the military, or for cardiovascular operations, or in dentistry. The per unit cost varies from about INR 60 to INR 3,000 depending on which market it is meant for. The bigger sizes, which go to the military, are in the range of INR 2,000-INR 2,500.
In the pipeline are plans to introduce these products in schools, colleges, and ambulances, through a road kit called the ASK Kit – Advanced Stop bleeding Kit.
Explains Leo, “It is a new initiative to introduce it to households. We don’t sell it through pharmacies yet, but we are working on that through partners such as providers to hospitals, colleges, ambulances, and industries, etc. We expect this product to come into retail in a couple of years but it still will take some time, since there are no advanced wound care products in the Indian market. So, it will be a little too early to introduce in pharmacies.”
On the funding side, it has raised Seed capital in 2010 from Ahmedabad-based venture capital firm GVFL and an undisclosed amount is its Series A funding last year from Accel Partners and IDG Ventures. Its team now comprises 50 people spread across Bengaluru, Ahmedabad, and its sales team in the rest of India.
The Challenge Of Building An Indian Brand
Surprisingly, while funding was not a challenge for Leo, what was more difficult was finding his way through the fuzzy regulatory network.
“In India, there are no clear medical device regulations which one can follow. So we were forced to look at US FDA and European Union regulations and comply with that and expect that Indian authorities will accept it. This big lack of clarity in terms of approval and absence of an authority to guide us were major challenges back then when we started in 2008. The situation has definitely improved quite a bit now from that time,” he explains.
Another major challenge was the not so great perception of India as a brand. He explains,
“When we go abroad or even in Indian markets, the doctors always look up to products developed outside. This perception is changing slowly but when we deal with life-saving products, it is always important to build out a brand. That’s the reason why we have spent quite a bit of time and money in building clinical endorsements. It would have been much easier if we had any competitor to look up to from India as a brand in medical device products.”
Additionally, there were procurement related hiccups. Government procurement, especially the tenders, are heavily skewed towards the incumbent companies. It takes a lot of persuasion to break into the major tenders, which acts as a major entry barrier for startups.
Nevertheless, there were also memorable milestones along the way which made the journey worth the wait.

Major Milestones
And one of them was landing the Indian Armed Forces (IAF) as a customer. Recounts Leo, “A lot of people discouraged us that they (IAF) don’t purchase Indian products or those manufactured by startups. But we are one of the few startups which could sell to them and make money. It gave us a lot of confidence and reputation because a product that can work with the Indian armed forces, in the field, gave a lot of confidence to doctors as well.”
Another milestone was the move to Bengaluru which aided faster growth. Developing a good team quickly was important and Axios’ current set of investors also helped in building enough traction quickly. Says Leo,
“The move helped us to turn ourselves quickly from a manufacturer into a brand. In the early stage, I was not keen on doing so fast, but I realised that a lot of companies stay in that bracket for a long period of time in India where they are happy with their manufacturing and small margins. But we wanted to build a brand.”
And it is that perception of building a brand changed the entire DNA of the company.
A Global Hemostatic Market Worth $3 Bn
Leo claims that Axio is growing around 3x per year and this figure coul leapfrog to almost double, next year. His belief stems from the fact that globally hemostatic market is about $3 Bn. A report by Research and Markets (Hemostat Market: India Industry Analysis and Opportunity Assessment 2016-2025) also concludes that the haemostat market is expected to register a CAGR of 5.8% from 2016 to 2025. India is about 10-15% of that market.
As per Leo, the product has a potential to hit $400 Mn-$500 Mn in the Indian market and the startup’s strategy is to get a pie of that market in the coming years. What also helps it, is the fact that while globally there are similar products just as QuickClot and Celox, developed by the US Army – in India, not many have heard the concept of hemostats.
He further adds, “Globally only three-four companies have attempted to do what we are doing. We are the probably the fourth/fifth company which has built a product pipeline for controlling bleeding in trauma. In India, we are very focussed on making this as the first intervention to bleeding. People should be looking at our products just like they say Xerox for photocopying.”
Thus, the idea is to target any and every field where there are frequent bleeding hazards. Hence, the startup does not want to limit itself to hospitals alone –from battlefields, to industries, and at some point of time, it aims to enter households. As the industry grows and the market matures, the idea is to be available at pharmacies. Which Leo explains is more a distribution game.
He adds, “There’s differentiation between B2B product and B2C product. When we look at the market we see an opportunity but the price points are still not attractive. You need to invest in building marketing and advertising. It is a little bit challenging to go to B2C directly given that we are building a new platform altogether. We already have a sizeable market in B2B. Once we are established in B2B, it will be easier to enter B2C.”
In the pipeline are also plans to build the brand in such a way so as to hit the market with an IPO in the next five years. The startup might also introduce this product in pharmacies abroad earlier as the price points are much more attractive over there. Leo explains that right now, in India, there will be a direct comparison with the low priced Band-Aid, hence he has made a strategic choice to stick to solely B2B at the moment.
Editor’s Note
There is no denying the fact that Axio has a huge first-mover advantage, as far as the Indian markets are concerned. While Axio has cemented its reputation by landing the Indian Army as a major client, how easily and how soon it is able to make inroads in the B2C markets will determine its next wave of success. Given the fact that India has a dismal record as far as road safety is concerned, seem more a necessity rather than a luxury to bring down the high mortality rate in road accidents.